Parylene conformal coating boasts a bevy of benefits and properties that make it an appealing choice for a variety of medical device applications.
Where it's Used
Parylene conformal coating boasts a bevy of benefits and properties that make it an appealing choice for a variety of medical device applications. Chief among parylene’s advantages for medical applications, however, is that it meets USP Class VI and ISO 10993 biocompatibility requirements — a characteristic that is essential for many critical medical products and that other types of conformal coating sometimes lack.
But the benefits don’t stop there. Parylene also provides:
And in the case of medical implants or medical devices that are inserted into the body, in particular, parylene performs double duty by protecting them from direct exposure to the harsh environment of the human body while protecting the human body from the foreign medical device. We highlight three such types of medical products that profit from parylene’s unique blend of properties.
Catheters
Catheters, which are inserted into the body for a variety of reasons, reap the benefits of parylene’s proven biocompatibility and barrier properties. However, parylene conformal coating most notably lends a low coefficient of friction that translates into enhanced lubricity to these medical devices. Imparting lubricity nearing that of PTFE, parylene conformal coating of catheters facilitates smooth navigation of the tortuous curves of the human body.
Cardiac-Assist Devices
When applied to electronic cardiac-assist devices such as pacemakers and implantable cardioverter-defibrillators (ICDs), parylene conformal coating encapsulates the sensitive medical electronics necessary for the life-critical implants to function. This parylene seal serves to protect the medical devices and their electronics from exposure to the potentially damaging and corrosive effects of bodily fluids, which can compromise performance or even cause medical device failure. Furthermore, the parylene coating creates a barrier between the implant and the body so that the body is not exposed to potentially dangerous materials and chemicals employed in the device. As a dielectric, parylene also forms an electrical barrier between the electronics inside the implants and the electrical signals generated by the surrounding body systems.
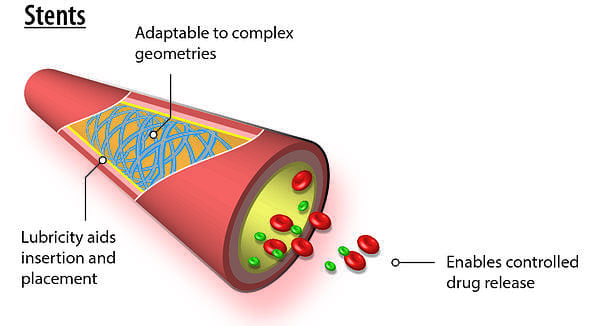
Stents
Because of the vapor-deposition polymerization process by which it is applied, parylene conformal coating is prized for being completely conformal and yielding a uniform thickness. These advantages are particularly useful in coating stents, which feature complex geometries. In addition, parylene’s lubricious nature aids in stent positioning while its corrosion-resistant properties help to protect the metal-based device from bodily fluids. Parylene has risen to further prominence in this field in recent years by enabling the controlled release of drugs as well as by acting as a bonding agent or tie layer on drug-eluting stents. In this latter capacity, the parylene coating is leveraged because it adheres well to both the bare metal stent and the therapeutic agent.
For Medical Devices
Parylene (XY -- poly(para-xylylene)) organic polymers are highly regarded through a wide range of industries – aerospace/defense, automotive, commercial, industrial, medical – for their utility as conformal coatings. Chemically inert, colorless, linear/polycrystalline and optically clear, XY coatings provide exceptional barrier protection, dielectric reliability, and insulation for printed circuit boards (PCBs) and similar electronic assemblies whose components must maintain performance through all operating conditions. Parylene conformal films safeguard function in the presence of biogases, biofluids, chemicals, moisture/mist, salt compounds, and temperature fluctuations.
Applied by a unique chemical vapor deposition (CVD) process, parylene assumes a gaseous consistency, allowing it to seep deep within a substrate surface while simultaneously forming an effective outer layer of protective film. The result is a truly conformal, pinhole-free coating, absent extraction issues, leaching and outgassing. CVD synthesizes coating in-process, allowing the deposited film to:
CVD lets XY penetrate and coat small cracks, crevices, and openings along, within and under the assembly’s surface, reaching even hidden component areas, places where liquid coating materials – brushed, dipped or sprayed – cannot effectively approach.
Clean, self-contained CVD requires no additional chemicals to complete the process, depositing uniform XY film thickness, even on irregular surfaces. Vapor methods assure reliable coating deposition on an expanded range of devices and products, with longer-lasting security and performance, minimizing the need for repair and potential of assembly failure.

Sterilization Processes for Parylene Conformal Films
Sterilization procedures for industrial processes destroy, permanently deactivate or otherwise remove all lifeforms existing on or within products, many designated for biomedical use. The objective is to establishing exceptional product safety, eradicating all sources of existing or potential contamination. Sterile pharmaceutical products include medical implants of all kinds, which need to function without fail in often turbulent biomedical environments.
As with XY’s superior comparative worth in relation to liquid coatings, it also registers well for sterilization. Chief among the coating’s benefits is the ability to withstand common sterilization techniques -- steam autoclave, electron beam (e-beam), ethylene oxide (EtO), gamma radiation and hydrogen peroxide ((H2O2)) plasma.
The selection of the appropriate sterilization process is crucial to the success of the assignment. Selection criteria emphasize the type of device being sterilized, its purpose, and the type of XY coating being used. CVD allows parylene application to most vacuum-stable materials -- ceramics, fabrics, granular materials, metals, paper, plastics. Across type, XY varietals withstand the sterilization methods described above; different XY material types respond better to specific sterilization techniques.
Parylene withstands all common sterilization methods, but matching XY type with sterilization process is necessary. While autoclave is not recommended for N or C, radiation techniques are successful. H2O2 plasma sterilization treatment slightly alters C’s dielectric strength but does not for N. Radiation dosage and procedural duration always need to be monitored for optimal results. With high thermal stability, F better withstands autoclave temperatures recommended for sterilizing multi-use implants.
Superior Protection
Biocompatible parylene conformal coatings provide superior protection for medical stents. They represent an enabling technology consistently applied to medical devices of all types for 35 years, to diminish problems stemming from surface microporosity and consequent biofluid corrosion after implant. Providing a reliable barrier to chemicals and moisture, parylene’s static and dynamic coefficients of friction are comparable to those of Teflon.
Implanted parylene devices have demonstrated:
Applicability of Parylene for Stents
Chemically inert, parylene provides a superior conformal coating for stents, among the most valuable of implantable medical devices. Its use meets all professional requirements, including the USP Class VI implantable plastic material standard, conforming to both the ISO 10993 biocompatibility standard and RoHS standards.
Medically-implanted stents require a finely-conceived and produced geometrical configuration to be successful. Parylene adapts to these product requirements; its appropriate conformability characteristics result from its unique chemical vapor deposition (CVD) application process. Enacted in a specialized vacuum chamber, CVD:
CVD processing also eliminates formation of undesirable air pockets along and within the coating’s surface, a major drawback of liquid coatings such as acrylic, epoxy, silicone and urethane. With parylene, there is neither bridging nor over-filming of minute surface openings, a drawback commonly occurring with wet coating materials. Since parylene generates a gaseous, molecule-by-molecule growth that adheres to the stent’s every contour, the truly conformal coating that results penetrates the stent’s surface microcrevices, and prevents corrosion from harsh bodily fluids after deployment. In addition, the galvanic electrochemical reaction that can develop when dissimilar metals like nitinol and platinum are combined within a single stent are largely eliminated by parylene’s excellent moisture barrier properties, improving the stent’s function while prolonging its operating life.
In addition, parylene’s micron-thin coatings offer this exceptional resilience and protection to implanted stents without adding significant dimension or weight. This feature is an added benefit for internal body medicine and can be easily adapted for MEMS/nano purposes. Present research and development focused on controlling coating dimension and weight should lead to the creation of stents that are simultaneously smaller and more complex, with enhanced capacity for medical treatment. Only CVD-applied parylene generates these specialized service features.
Among other advantageous properties for use with stents, parylene conformal films:
Parylene conformal films can be precisely deposited on virtually all available stent materials, simultaneously serving as both a carrier for drug delivery and a mechanism for controlled pharmaceutical release.
For widely-used drug-eluting stents, parylene films significantly diminish the incidence of restenosis, the re-narrowing of arteries, for most patients. Typically, treatment drugs are applied over a parylene-coated stent substrate. This pharmaceutical layer is then treated with another coating of micro-thin, partially porous parylene, which permits its introduction into the patient’s system at a regulated level. In some cases, drugs and parylene are deployed in multiple, alternative layers for longer-term treatment effectiveness.
Future Improvements Inevitable
Parylene is well-suited for use with in vivo devices such as stents. Coating virtually any surface that is vacuum-stable conformally and uniformly through CVD, parylene stands out among conformal materials as a superior coating for stents’ often intricate structures, achieved without pooling or webbing. Its versatility continually leads to new applications:
This combination of medical benefits and healthcare cost savings coupled with a lower per-unit cost will likely stimulate further development of better quality stents, protected by parylene conformal coatings.
Implantable Medical Devices and the Uses of Parylene
In surgery, materials grafted or inserted onto or within a bodily structure or a functioning organ, are considered implanted. Although a medical implant may be composed of such bodily tissue as a blood vessel or tendon, many are made from artificial substances, surgically positioned within the body to improve the patient's health by upgrading the performance of affected organ or structure.
Parylene conformal coatings are linear and polycrystalline substrate coverings that are also highly biocompatible and integratable within the human body. Also chemically inert, colorless and clear, their lack of pigmentation minimizes possible leakage into the bodily system during use, protecting from potential infection or irritation of the blood stream and internal organs.
Chemical Vapor Deposition
Parylene coatings differ from other commercial conformal coatings. They rely on a chemical vapor deposition (CVD) process rather than wet techniques -- dipping, spraying, or brushing -- for coating application.
Parylene's vapor-phase, chemical-vacuum polymerization process converts powdered parylene into a gaseous form at the molecular level. Reheated to high temperatures, the vapor reverts to a single molecule construction that simultaneously coats all parts of an assembly or component as a durable and transparent, polymerized film.
 These features promote exceptional crevice/multi-layer penetration for all components and instruments, as well as dependable levels of dry-film lubricity. The superior coating is uniform, pinhole-free, and highly dielectric, without the bridging, puddling or run-off common to conformal coatings using wet application processes. Its capacity to deliver ultra-thin yet resilient coatings makes parylene ideal for most of the MEMS/nano technologies increasingly used for medical implantables.
These features promote exceptional crevice/multi-layer penetration for all components and instruments, as well as dependable levels of dry-film lubricity. The superior coating is uniform, pinhole-free, and highly dielectric, without the bridging, puddling or run-off common to conformal coatings using wet application processes. Its capacity to deliver ultra-thin yet resilient coatings makes parylene ideal for most of the MEMS/nano technologies increasingly used for medical implantables.
The Benefits of Parylene-Coated Implantables
Since CVD-applied parylene coatings adhere in a device-specific format to the entire range of vacuum-stable materials and devices, surface coverings conform explicitly to the contours of the substrate. In addition to biocompatibility, parylene coatings are biostable, inert, non-toxic and meet FDA compliance guidelines, increasing antimicrobial protection during extended use. These properties assure protection of coated implantables through a diversity of extreme operating conditions typical to internal medical component usage, ensuring implanted devices perform as designed for greater periods of time.
Primary implants of this type include:
These devices represent just a sampling of the growing list of efficient parylene-protected and assisted implantable medical applications.
Conclusion
Implantables coated with parylene have repeatedly demonstrated their reliable utility for function-focused internalized medical devices.
Instruments coated with parylene can be safely situated in less accessible, more-constricted regions of the body, difficult-to-reach places often requiring critical monitoring and care. These segments of the body are precisely those where implantable devices are most needed. Ultra-thin coating layers provide superior component protection, adding only minimal mass to the device, extending MEMS/nano applications. Parylene's low dissipation factor supports improvements of both device performance and patient treatment, by extending the implantable's durability and functional capacity.
Parylene coating's use for implantable devices includes CADs, catheters, electrosurgical devices (ESUs), and stents, The value and use of parylene-protected medical instruments and procedures will proliferate, as technology develops. New ways of applying parylene continue to be produced to coincide with technological expansion in the medical industry; new varieties of parylene are similarly evolving to match these developments.
How Parylene Helps
Coronary stents are tubular medical implants that serve as a scaffold to open clogged or narrowed arteries in an effort to increase blood flow and reduce the potential for adverse cardiac events such as heart attacks. And providing critical support to these support structures is parylene conformal coating.
Parylene use in stents has revolutionized the efficacy and capabilities of these life-changing medical implants. The novel conformal coating not only facilitates a smooth delivery of the medical device to its destination, it also provides a valuable vehicle for drug delivery.
The Parylene Deposition Process
In contrast to other conformal coatings that are brushed, dipped, or sprayed onto a substrate, parylene is applied via a vapor-deposition process in a vacuum chamber. During this vapor deposition polymerization process, raw dimer in powder form is subjected to high heat and, in turn, transforms into a gaseous monomer without an intermediate liquid stage. This gas is then deposited one molecule at a time onto the desired substrate in the coating chamber at ambient temperatures. Finally, the cold trap phase cools temperatures down to a range of -90° to -120°C in order to remove residual parylene.
This unique vapor-deposition method allows parylene to adequately penetrate and coat extremely small stent features. In addition, this process enables the uniform and consistent coating of the complex geometries that characterize the life-saving devices. Deposited in a 0.5-micron-layer thickness, parylene is also pinhole free and can fully coat inside crevices and contours as small as 0.001 mm. Parylene is impermeable upon deposition to a thickness of 1.4 nanometers.
Unlike some other conformal coatings, parylene doesn't adhere chemically to surfaces—it adheres mechanically. This characteristic is beneficial for parylene use in stents, as it promotes the effective coating of a wide range of substrates. In fact, parylene has been documented to coat everything from silicon to paper, from steel to a bird feather. Thus, parylene's ability to coat common stent materials such as stainless steel and nitinol is well documented.
Is Parylene Safe for Stents?
Parylene is an ideal conformal coating choice for stents, which are implantable medical devices, because it is extremely inert when inside the human body. It meets the USP Class VI implantable plastic material standard and conforms to RoHS standards, as well as to the ISO 10993 biocompatibility standard.
In addition to being inert and hydrophobic, parylene offers dry-film lubricity, which is particularly advantageous during device delivery and implantation. It features a coefficient of friction nearing that of Teflon, although it has significant benefits relative to Teflon, or PTFE, including a lack of toxic outgassing. Parylene is also clear, while Teflon is either milky or grainy in appearance.
Parylene use in stents serves multiple functions, ultimately helping to facilitate a smooth delivery while reducing irritation and inflammation.
Parylene Use in Drug-Eluting Stents
Despite the overall success of early stents, a sizable number of patients experienced restenosis, or the re-narrowing of arteries. And while coating stents with therapeutic agents was identified as a solution to this dangerous phenomenon, the solution brought its own technological challenges in terms of bonding drugs to the stent structure and controlling drug release.
Parylene use in stents has been key to overcoming these challenges, however, and making today's highly effective drug-eluting stents possible. First, stents were coated with parylene to give them a surface onto which drugs such as Sirolimus could be applied. The next step was to cover the drug-impregnated layer with another coating of parylene. This second coat is designed to be thin and partially porous, allowing the drug to pass through at a controlled rate of release. Some stents even use a series of multiple layers of drugs and parylene barriers for better long-term effectiveness.
As in other medical devices, parylene use in stents improves their effectiveness and, more importantly, patient outcomes. The conformal coating can be accurately deposited on any commonly used stent material and can serve both as a carrier for drug delivery as well as a mechanism for controlled release. Its capabilities in stents point to opportunities to create additional parylene-based drug-eluting technologies in the future.
How it Protects
Whenever implantable devices come into contact with the human body, long term protection against body fluids, enzymes, proteins, and lipids is vital. Bio-medical surfaces typically require coating to protect from moisture, chemicals, and other potentially harmful substances.
A downfall for wet chemistry, liquid coatings such as silicones, acrylics, epoxy, or urethanes is that they do not meet bio-compatibility requirements and cannot be applied with precise control. On the contrary, parylene does not out-gas and is very effective against the passage of contaminants from both the body to substrate or substrate to body.
Parylene can be applied a couple of mils thick to a few hundred angstroms in thickness, it is pin hole free, applied at room temperature, contains no additives, insoluble in most solvents, very lubricious, has a very high dielectric strength, and is biocompatible and bio-stable.
Parylene C is currently being used is a number of well documented bio-medical implantable devices. Parylene C has been proven to be a terrific biocompatible material. It is USP Class VI implantable plastic material and conforms to material ISO-10993 Biological Evaluations for Medical Applications. Parylene C is also probably the longest proven protective biocompatible material.
Sources:
Parylene Technology for Neural Probes Applications. Changlin Pang. California Institute of Technology. 2008.
S. Nancy, "Literature Review: Biological Safety of Parylene C," Medical Plastics and Biomaterials, vol. 3, pp. 30-35, March 1996.
B. Humphrey, "Using Parylene for Medical Substrate Coating," Medical Plastics and Biomaterials, Janurary 1996.
If you're looking for parylene coating services for medical devices, contact Diamond-MT today. Get started by calling us at 814-535-3505 or completing a quote request.